
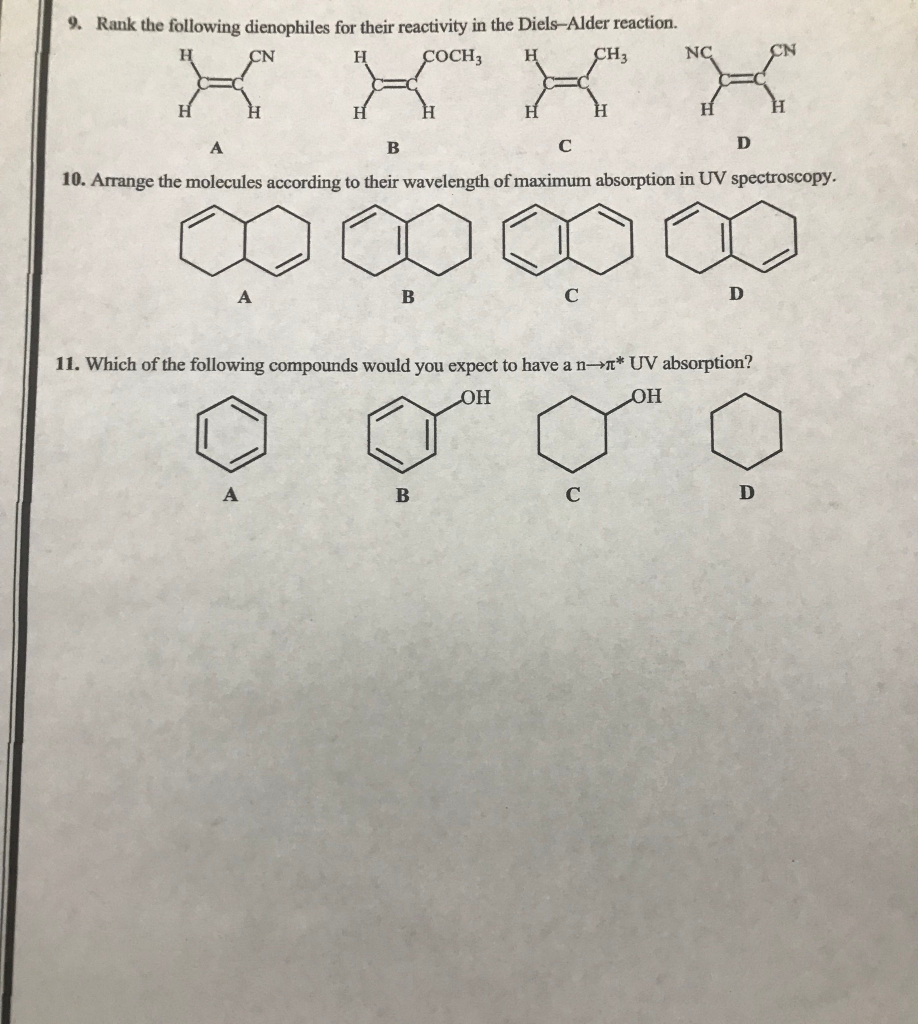
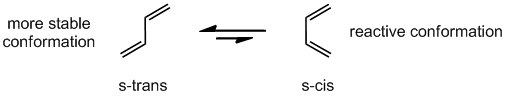
Īn AMI semiempirical method was used to investigate the Diels-Alder cycloaddition reactions of vinyl sulfenes with buta-1,3-dienes.156 The reactivity and stereoselectivity of vinyl boranes have been reviewed.157 Aromatic methyleneamines undergo reverse-electron-demand Diels-Alder reactions with cyclopentadiene, norbom-ene, and vinyl sulfides.158. The 4 + 2-cycloadditions of A-acylthioformamides as dienophiles have been reviewed. A density functional theoretical study of the hetero-Diels-Alder reaction between butadiene and acrolein indicates that the endo s-cis is the most stable transition structure in both catalysed and uncatalysed reactions.The formation and use of amino acid-derived chiral acylnitroso hetero-Diels-Alder reactions in organic synthesis has been reviewed. Ab initio calculations of the Diels-Alder reactions of prop-2-enethial with a number of dienophiles show that the transition states of all the reactions are similar and synchronous.Thio- and seleno-carbonyl compounds behave as superdienophiles in Diels-Alder reactions with cyclic and aryl-, methyl-, or methoxy-substituted open-chain buta-1,3-dienes.The intramolecular hetero-Diels-Alder reactions of 4-benzylidine-3-oxooxathiolan-5-ones (100) produce cycloadducts (101) and (102) in high yield and excellent endo/exo-selectivity (Scheme 39). AAI9717067.Buta-1.3-diene Diels-Alder reactions withĪn extensive review of the hetero-Diels-Alder reactions of 1-oxabuta-1,3-dienes has been published. Dissertations (1962 - 2010) Access via Proquest Digital Dissertations. Shang, Lewei, "Studies on the reactivity of tricarbonyl diene and dienyl iron complexes" (1996). Only if the two adjacent hydroxide groups which are conformationally flexible to coordinate the periodate can cleavage occur. The reactivity of glycol tricarbonyl iron complexes toward to sodium periodate depends upon the flexibility of the conformation of the complexes. Thus glycol-diene iron complexes may be cleaved under NaIO$\sb4$ conditions. Tricarbonyl iron complexes, which have been found to be unstable under oxidation conditions in certain cases, have been found to be reasonably stable to sodium periodate. Thus cations 24, which is more stable/less reactive, reacts with greater selectivity than does cation 3a which is less stable/more reactive. The difference is rationalized on the basis of the "reactivity/selectivity" principle. In contrast, reaction of the Fe(CO)$\sb3$ complexes cation (3a) proceeds via attack at both of the pentadienyl termini (C1, C5) and at the internal positions (C2, C4). malonate anion, NaBH$\sb3$CN) proceeds via attack at the substituted pentadienyl terminus in a highly regioselective fashion. Reactions of the Fe(CO)$\sb2$PPh$\sb3$ complexes pentadienyl cations (24) with "soft" nucleophiles (i.e. This reversible process could be utilized for the generation of optically pure dienyl phosphonium salts via desymmetrization. Steric interactions between the phosphine and other substituents on the dienyl moiety may destabilize the phosphonium salt such that phosphine dissociation can occur at an observable rate. The reversible addition of phosphines to certain (pentadienyl)Fe(CO)$\sb3$ has been observed. The use of organoiron complexes has been recognized as a powerful tool in synthetic methodology due to their ability to react with nucleophiles and electrophiles in regioselective or stereoselective fashion. Studies on the reactivity of tricarbonyl diene and dienyl iron complexes


 0 kommentar(er)
0 kommentar(er)
